By Amanda Saratsis, MD | December 1, 2023
Over the past decade, invasive techniques for monitoring and diagnosing cancers have been replaced with non-invasive techniques. One example is an emerging concept known as liquid biopsy which is helping to revolutionize the field of oncology. It offers particular benefit in caring for patients with hard-to-treat cancers, including brain tumors and sarcomas.
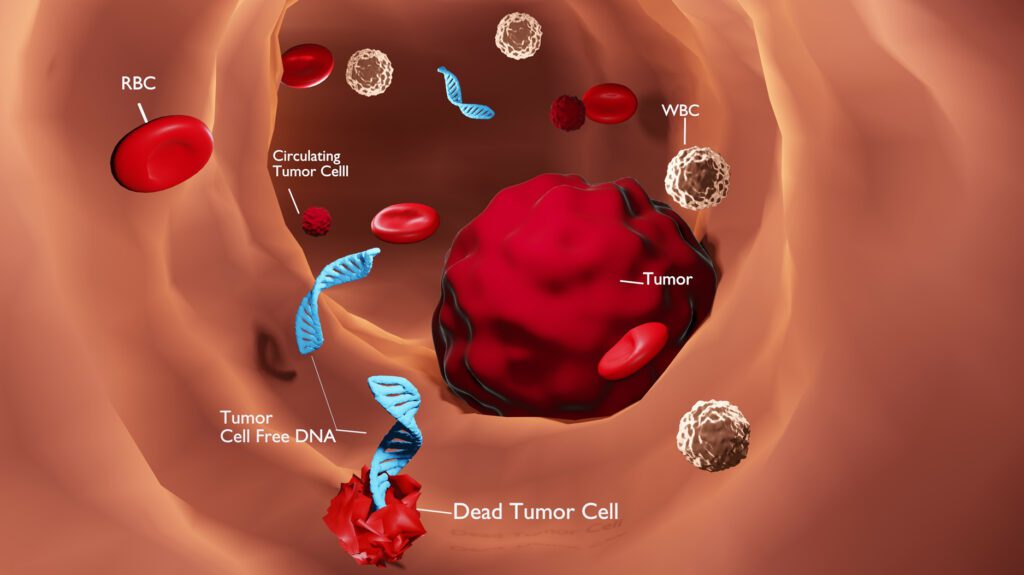
Assessing Solid Tumors With a Blood Test
Our blood and bodily fluids carry tiny amounts of DNA from broken-up cells. If we have cancer, some of that DNA comes from tumor cells. Over the past ten years, technologies have been developed that enable the detection and even quantification of this tumor DNA, also known as cell-free DNA (cfDNA) or circulating tumor DNA (ctDNA), in patients with a variety of different cancers. As a result, cfDNA or ctDNA profiling, or liquid biopsy, has emerged as a valuable, non-invasive tool for evaluating tumor genetics and even for tumor diagnosis and disease monitoring.
Advances in tumor diagnosis, neurosurgical techniques and development of multimodal therapies have collectively led to an increase in patient survival for children with brain tumors. However, mortality due to pediatric central nervous system (CNS) tumors remains high and toxicity from treatment can have long term effects. Unlike other pediatric cancers such as Leukemia, where molecular biomarkers can be used to measure residual disease and response to therapy, monitoring pediatric brain tumors is done predominantly radiographically. It is often difficult to use this data to guide therapy. As a result, there is a significant need for discovery of biomarkers of this disease to improve clinical outcomes and quality of life for these children.
Excitingly, over the past decade there have been a plethora of studies that demonstrate the feasibility of tumor biomarker detection via liquid biopsy. This includes detection and quantification of circulating tumor cells, cell-free DNA (cfDNA) and even cell-free RNA (cfRNA) in blood, urine or cerebrospinal fluid from patients with a variety of cancer types.
This detection can be used for diagnosis, monitoring response to treatment, and even cancer screening in some adult cancers, including lung, breast, colon, gastric and bladder. The first liquid biopsy assay was approved by the FDA in 2016 and can be used to determine lung cancer patient sensitivity to targeted therapy. Since then, several assays have been developed for a range of cancer types and a few are even under development for early cancer detection.
Unlocking Answers
The work of Pediatric Cancer Research Foundation-funded researcher Dr. Brian Crompton helps illuminate the progress and the promise. Together with colleagues at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and elsewhere, Dr. Crompton is studying liquid biopsies in pediatric solid tumors. The researchers hope that these blood tests will eventually improve early detection, choice of treatment and monitoring of young patients with these diseases without having to sample the tumor itself.

Dr. Brian Crompton
In the last year, this team has shown that circulating tumor DNA (ctDNA) can be detected and measured in patients with five of the most common pediatric solid tumors: Ewing Sarcoma, Osteosarcoma, Neuroblastoma, Alveolar Rhabdomyosarcoma and Wilms’ Tumor.
The scientists also found early correlations between ctDNA liquid biopsy findings and cancer progression in two pediatric bone cancers, Ewing Sarcoma and Osteosarcoma. Liquid biopsies potentially could aid in tailoring treatments for both bone cancers — for example, by helping to predict which young patients will respond well to treatments, which cannot be done with current tools.
Of course, applying liquid biopsies to pediatric solid tumors requires developing new approaches. “We can’t just take adult assays and apply them to pediatrics, because the patterns of genetic mutations we see in children with cancer are completely different than the patterns of mutations we see in adults,” Dr. Crompton explains.

Lessening Side Effects, Detecting Recurrence
Importantly, there is a growing body of evidence to support the use of cfDNA profiling for pediatric brain tumors, the second most diagnosed of all childhood cancers. While there are technical challenges associated with detecting biomarkers from children with brain tumors compared to those with blood cancers (Figure 1), markers for gliomas including difuse midline gliomas, medulloblastomas, ependymomas and even atypical teratoid rhabdoid tumors (ATRT) have all been detected. In some cases, trends in the amount of cfDNA detected can reflect disease burden and response (or resistance) to therapy and therefore has great promise for guiding therapy. Multiple clinical trials of a variety of chemotherapeutic agents for pediatric brain tumors now incorporate blood and/or cerebrospinal fluid monitoring (via lumbar puncture or ventricular sampling) for biomarker analysis.
As a result, there is great hope on the horizon for this technique of liquid biopsy to improve care and clinical outcomes for these children. Researchers believe that we will one day be able to use liquid biopsy to identify patients who could be cured with reduced-intensity therapy, which could lessen the risk of long-term side effects. They are also optimistic that these assays could be used to monitor patients for early signs of relapse, when treatment may be more successful, and even for early detection of malignancies in families at high risk of developing cancer.
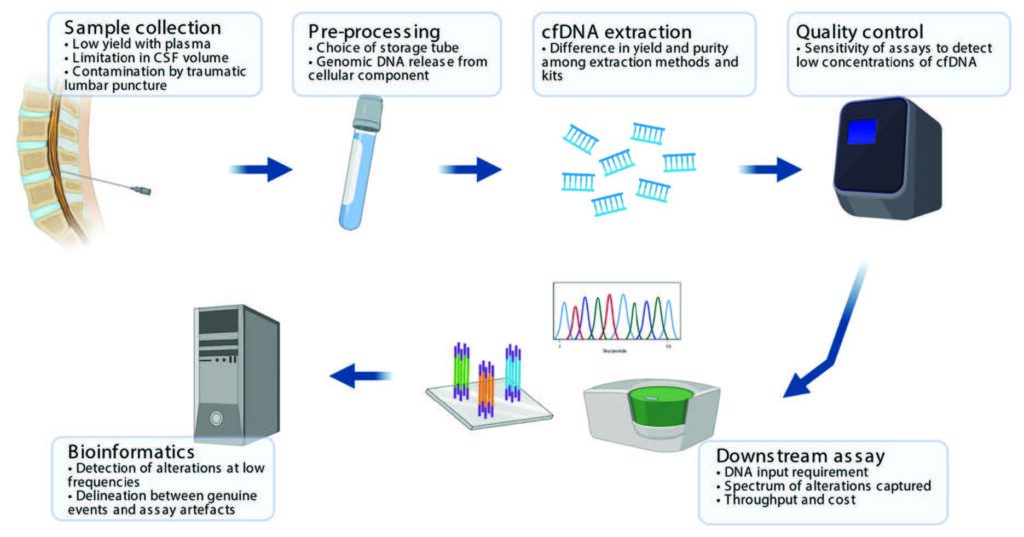
Figure 1. Summary of technical considerations. The application of cfDNA profiling for children with primary CNS tumors requires optimization of sample handling and experimental pipeline, inclusive of wet and dry lab components. Source: Lie A et al, Circulating tumor DNA profiling for childhood brain tumors: Technical challenges and evidence for utility. Lab Invest. 2022 Feb;102(2):134-142.




